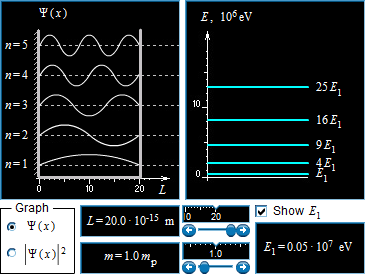
The graphs also show a different value for the value of n which, the probability of finding the particle in that area is much higher as the values of n rise. As the length increases and decreases there are many changes that occur to these probabilities. The longest wavelength with a standing wave L is on that goes to twenty times ten to the negative fifteenth meters and the mass at the minimum must be 1.0 times the mass of a proton.
 This is the energy of the ground state this can also be written as E = h^2/8mL this is the equation derived and given for an atom in ground state for a particle in a box. As the length of the box in this denoted as L increases then the value of the energy is decreased. It is inversely proportional to the ground state energy of an atom.
This is the energy of the ground state this can also be written as E = h^2/8mL this is the equation derived and given for an atom in ground state for a particle in a box. As the length of the box in this denoted as L increases then the value of the energy is decreased. It is inversely proportional to the ground state energy of an atom.
In this photo the value of the length of the standing waves has not been changed however the mass has been increased. Instead of being only the mass of one proton, the mass is now increased to five protons. This value makes the energy much lower. When the energy levels are higher the amount of energy is not nearly as high as any of the other energies as the mass is increased. Basically the higher the mass the lower the energy weirdly.
No comments:
Post a Comment